ReSure Sealant is indicated for intraoperative management of clear corneal incisions (up to 3.5mm) with a demonstrated wound leak for which a temporary dry surface can be achieved, in order to prevent postoperative fluid egress from such incisions following cataract surgery with intraocular lens (IOL) placement in adults.1
To learn more, visit resuresealant.com.
Please see Important Safety Information below.
Benefits of ReSure Sealant
Eliminates the need for post-op follow-up visits for suture removal.2
Wound sealant in complex* or non-complex cases.2,3
Eliminates potential complications related to suture.2
*In the pivotal trial, ReSure Sealant was not studied in complex cases.
Dr. Terry Kim showing the application of ReSure Sealant following cataract surgery
WARNINGS
ReSure Sealant should not be used on actively leaking incisions in which a temporary dry ocular surface cannot be achieved.
Do not use in patients who are allergic to FD&C Blue #1.
PRECAUTIONS
ReSure Sealant is for single-use only; discard open and unused product. Use within 30 minutes of removing the mixing tray from foil pouch.
Prior to application, ensure incision site is not actively leaking; remove any standing moisture from the surrounding ocular surface and incision and ensure the site is dry.
Prophylactic use of ReSure Sealant on corneal incisions without intraoperative leakage was not evaluated in the clinical study.
ReSure Sealant will not replace the need for sutures in certain circumstances, including the need for long-term mechanical support to the incision.
ADVERSE REACTIONS
The most commonly reported (≥1%) ocular adverse events that occurred in patients treated with ReSure Sealant in the pivotal trial included: worsening best corrected visual acuity (7%), increase in intraocular pressure (5%), corneal astigmatism (3%), eye pain (3%), posterior vitreous detachment (2%), and anterior chamber inflammation (1%).
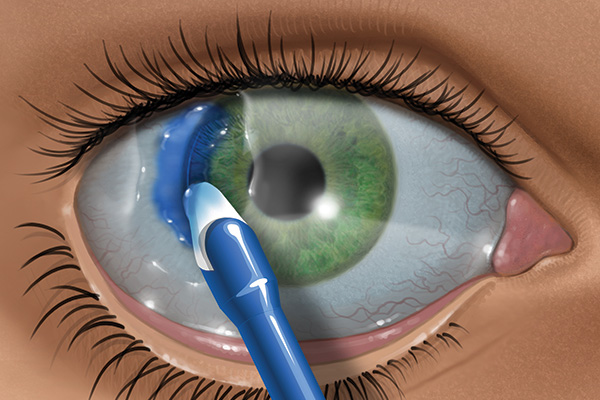
Application of ReSure Sealant1
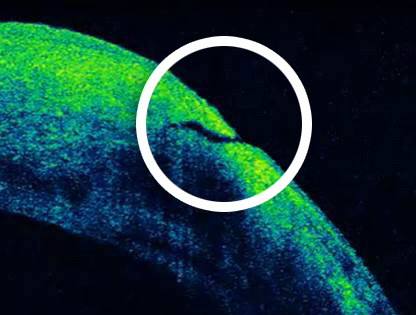
Primary unsealed clear corneal incision.
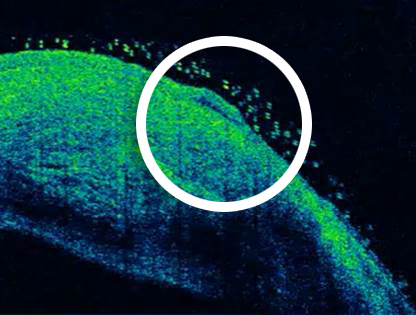
ReSure Sealant selectively adheres to the de-epithelialized tissue and seals the wound.2
Images courtesy of Deepinder K. Dhaliwal, MD, LAc, University of Pittsburgh Medical Center.
REFERENCES: 1. ReSure Sealant [Instructions For Use]. Bedford, MA: Ocular Therapeutix, Inc.: LCN 80-1004-011 Rev C. 2. Masket S, et al. J Cataract Refract Surg. 2014;40(12):2057-2066. 3. Nallsamy N, et al. J Cataract Refract Surg. 2017;43:1010-1014. .

