DESIGNED TO ADDRESS THE ISSUE OF PATIENT NON-COMPLIANCE WITH EYE DROPS
In preclinical and early clinical trials, OTX-TIC exhibited an acceptable safety profile, maintenance of drug levels in the aqueous humor, and a sustained lowering of intraocular pressure.1
EXISTING TREATMENTS
- High rates of non-adherence to glaucoma therapies2-4
- Poor adherence has been shown to be associated with disease progression and vision loss5,6
- Ocular hyperemia7
- Life-long daily burden of patient administration8
PRODUCT CANDIDATE ATTRIBUTES
- Travoprost-loaded microparticles embedded in hydrogel1
- Administered with 27G or 26G needle9
- Resides in the iridocorneal angle1
- Fully biodegradable1
Caution: OTX-TIC is currently undergoing clinical evaluation and is limited by law to investigational use only. This product has not been approved by the FDA as safe or effective.
Intracameral injection of OTX-TIC

Injection of OTX-TIC implant in the anterior chamber of the eye.
Implant drops down and resides in the iridocorneal angle.
About Glaucoma
Glaucoma is a disease that damages your eye’s optic nerve.10 It usually happens when fluid builds up in the front part of your eye, thereby increasing intraocular pressure (IOP).10 Elevated IOP is associated with damage to the optic nerve, which may result in irreversible vision loss.10 Glaucoma is the second leading cause of blindness in the world.11 The Glaucoma Research Foundation estimates that over three million Americans have glaucoma.12 To lower IOP, physicians typically initiate treatment by prescribing drugs administered as eye drops.13 The classes of topical drugs used to treat glaucoma include prostaglandin analogs, or PGAs, beta-blockers, alpha-adrenergic agonists and carbonic anhydrase inhibitors.14,15 These drugs decrease IOP by either decreasing fluid production or enhancing fluid drainage.14 PGAs are the most widely prescribed class of drugs for glaucoma and are considered first-line glaucoma treatment.16,17 PGAs reduce IOP by enhancing the clearance and drainage of ocular fluid.14 The most frequently prescribed PGA is once-daily latanoprost, although travoprost and bimatoprost are also frequently used in the management of open-angle glaucoma.16 In cases where glaucoma is not easily managed by a drug regimen, surgical or laser treatments may be undertaken.13
According to IMS Health data, approximately 34.8 million prescriptions were filled in the United States in 2020 for drugs administered by eye drops for the treatment of glaucoma, resulting in sales of approximately $3.2 billion.18 A typical prescription provides approximately one month of treatment. We expect prescription volume to grow, in large part as a result of the aging population. According to IMS Health, PGAs accounted for approximately half of the prescription volume in the glaucoma market in 2020.18
Implant Visualization1
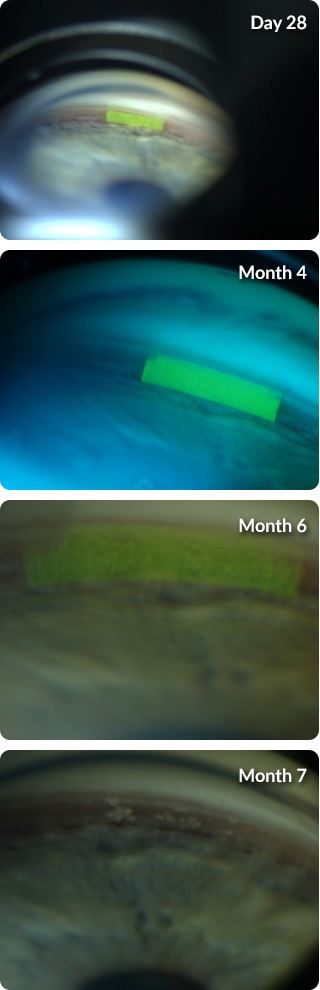
OTX-TIC visualized in a patient through a slit lamp in the Phase 1 clinical trial.
REFERENCES: 1. Walters TR, et al. Presented at the ASCRS Annual Meeting; Boston, MA; May 16, 2020. 2. Olthoff CM, et al. Ophthalmology. 2005;112:953-961. 3. Schwartz GF, et al. Surv Ophthalmol. 2008; 53(suppl1):S57-S68. 4. Nordstrom BL, et al. Am J Ophthalmol. 2005;140:598-606. 5. Sleath B, et al. Ophthalmology. 2011;118:2398-2402. 6. Newman-Casey PA, et al. Ophthalmology. 2020;127(4):477-483. 7. Zimmerman TJ, et al. Presented at the ARVO Annual Meeting; Fort Lauderdale, FL; May 6-10, 2007. 8. Kaur D, et al. J Curr Glaucoma Pract. 2012;6(1):9-12. 9. Data on file 017. Ocular Therapeutix, Inc. 10. Weinreb RN. JAMA. 2014;311(18):1901-1911. 11. Quigley HA, et al. Br J Ophthalmol. 2006;90(3):262-267. 12. Friedman DS, et al. Arch Ophthalmol. 2004;122(4):532-538. 13. Primary Open-Angle Glaucoma PPP 2020. American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-ppp. Published January 5, 2021. Accessed February 2, 2021. 14. Gupta D, et al. Am Fam Physician. 2016;93(8):668-674. 15. Li T, et al. Ophthalmology. 2016;123(1):129-140. 16. Ophthalmic Glaucoma Agents. Multum Therapeutic Class Comparison, United States, 2018. https://clincalc.com/DrugStats/TC/OphthalmicGlaucomaAgents. Accessed April 24, 2021. 17. Whitson JT. Expert Opin Pharmacother. 2007;8(18):3237-3249.18. IQVIA Market Data Breakdown 2020.
